Diana Pearson
Writer’s Comment: I first became interested in fungi while taking an introductory biology class during my second year at UC Davis. The professor explained how difficult it was to treat human fungal diseases due to the genetic similarities between fungi and animals. I started thinking about how researchers combat fungal diseases, and I soon realized it was a topic that I could see myself investigating in my future career. So, when I was assigned a scientific review in my UWP 104E class, the topic of fungi was not far from my mind. I wrote my paper on the biochemistry of bioluminescent fungi because bioluminescence is an interesting and understandable topic to the general public. I hope that my paper will inspire others to learn more about the many aspects of fungi just as my introductory biology professor sparked my interest in the topic.
Instructor’s Comment: When I assign my UWP 104E Science Writing students a scientific review, we first discuss purpose and rhetorical structure, and then each student faces three initial challenges: (1) pick a topic and scope, (2) decide upon a line of inquiry, (3) and find relevant articles published in the journals. My 104E student, Diana Pearson, initially wanted to write her review on the feasibility of using bioluminescent plants as lighting sources in green buildings. Ms. Pearson began her library research, struggled, enlisted the help of UCD research librarian Ruth Gustafson, they both struggled, and then, alas, were forced to concede that green building with bioluminescent plants was not a viable topic for review. At present, no one is writing principle research papers about it. Frustrated but not foiled, Diana continued onward and eventually discovered that an alternate track of research on bioluminescence and something plant-like did exist–yet minus the green-ness and chlorophyll–that is, bioluminescent fungi. With equal tenacity, Diana went on to write a fascinating, rigorous, and progressive review on the science, still in its infant stages, of fungi producing light.
—Brad Henderson, University Writing Program
Introduction
Luminescence refers to the emission of light that does not derive its energy from the temperature of an emitting body. Instead, the emission of light comes from chemical changes or movements of subatomic particles. Luminescence is an important process commonly exploited for use in the sciences, as well as the entertainment business. For example, light emitting diodes (LEDS), commonly used as indicator lamps, emit light via an electrochemical reaction known as electroluminescence. Another mainstream product, glow sticks, also creates artificial luminescence which occurs when isolated substances in the sticks are combined to make light through a chemical reaction. Yet, humans are not the only ones who have used luminescence to their advantage. Light emission can be found naturally in living organisms around the world through a process called bioluminescence. Due to the mystery of fungi’s bioluminescent mechanisms and to fungi’s practical applications, this paper will explore bioluminescence in fungi, explaining how and why this type of bioluminescence occurs.
Discussion
The Evolution of Bioluminescence and Its Prevalence
Most bioluminescent species live in the sea, and it has been estimated that 60-80% of the fish species in the deep sea are bioluminescent (Björn and Ghiradella, 2008). The dominance of bioluminescence in the ocean can be attributed to factors such as stable environmental conditions allowing an uninterrupted evolutionary history, large portions of the habitat receiving little to no light, and interactions occurring between a large diversity of taxa (Haddock, Moline, and Case, 2010). Yet, bioluminescence is not merely a marine phenomenon.
Except for vascular plants and higher vertebrates, most phyla have at least one bioluminescent species (Bjorn and Ghiradella, 2008). Despite its prevalence, bioluminescence evolved separately amongst the phyla. In fact, bioluminescence evolved independently at least 30 separate times according to comparisons of the biochemical systems involved (Bjorn and Ghiradella, 2008). It is commonly believed that bioluminescence first appeared during the Cambrian explosion as a response to the appearance of free oxygen in the environment (Bjorn and Ghiradella, 2008). The free oxygen would form peroxides essential to the process of bioluminescence in most organisms, including fungal species.
Bioluminescence in Fungi
More than 64 different species of light-emitting fungi belonging to three distinct evolutionary lineages have been discovered within the past century (Desjardin, Oliveira, and Stevani, 2008). The parts of the fungi that luminesce vary among the fungal species. Both the mycelium and the basidiomes of various fungi have the ability to emit light; however, there are only three species known to emit light only from the basidiome: M. irritans, M. lamprospora, and M. sublucens (Desjardin et al., 2008). This suggests that the ecological roles among bioluminescent fungi vary between species.
There are many theories explaining the advantage of bioluminescence in fungi. Some researchers speculate that luminescent fungi attract invertebrates to aid in dispersal of spores (Bjorn and Ghiradella, 2008; Desjardin et al., 2008). Strong luminescence within the spores gives some support to the hypothesis, but since both luminous and non-luminous basidiomes can produce millions of wind-dispersed spores per night, the significance of supplemental insect dispersal could be negligible (Weitz, 2004). Another hypothesis is that luminescence serves as a warning to repel predators and parasites interested in the fungi (Desjardin et al., 2008; Weitz, 2004). As of 2008, no studies had been conducted testing this theory (Desjardin et al., 2008). The most compelling argument for bioluminescence in fungi is that light emission is simply a byproduct of its oxidative reactions (Desjardin et al., 2008). Several studies have shown that bioluminescence does not require much energy and that the fungi may be releasing light (not heat) as an energy by-product of enzyme-mediated oxidation (Desjardin et al., 2008; Weitz, 2004).
Biochemistry of Fungal Bioluminescence: Early Findings
Early studies to discover the biochemistry of fungal bioluminescence applied Dubois’s classic “cold” and “hot” extract procedure (Desjardin et al., 2008). Dubois’s protocol came from his study in 1885 on the bioluminescence of the mollusk Pholas dactylis. A cold-water extract of the mollusk emitted light for several minutes following extraction. A hot-water extract from another mollusk added to the cold-water extract would also emit light; however, the hot-water extract alone could not produce light. Dubois concluded that the bioluminescent reaction required two separate chemical components and that the fuel component of bioluminescence could withstand heat, unlike the catalyst. It is now known that the hot-water extract contained luciferin, a small substrate, while the cold-water extract contained luciferase, a large enzyme. Despite Dubois’s findings, the first attempts at repeating his results were unsuccessful. It was not until 1959 that results from Airth and McElroy’s experiments matched Dubois’s original findings.
Airth and Foerster’s Hypothesis: A Two-Step Mechanism
In Airth and McElroy’s experiment, dried mycelium cultures of A. mellea were obtained by preparing the homogenate in a phosphate buffer (Airth and McElroy, 1959; Desjardin et al., 2008). This was followed by centrifuging and heating the supernatant in boiling water. The cold extract was obtained from dried mycelium cultures of C. veltipes. The cold-water extract was separated by centrifugation at 3000 g and again at 198,000 g, producing a soluble protein fraction and a membrane protein fraction. As the hot extract mixed with the soluble protein fraction in the presence of NADPH, light emission occurred. Without the presence of NADPH, light emission did not occur. This provided evidence that the soluble protein fraction contained a NADPH-dependent reductase while the membrane protein fraction contained the fungal luciferase.
Three years later, Airth worked with Foerster to propose a two-step mechanism for the chemical pathway for fungal bioluminescence (See Fig 1).
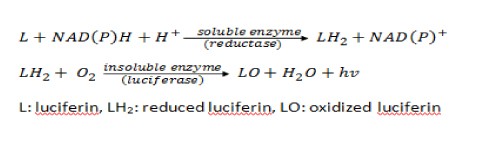
Figure 1. Airth and Foerster’s Proposal of the Pathway for Fungal Bioluminescence
The first step involves an initial reduction of the luciferin precursor found in the hot extract by an NADPH-dependent reductase, leading to the formation of the fungal luciferin (Airth and Foerster, 1962; Oliveira and Stevani, 2009). This is followed by the reaction of reduced luciferin with luciferase in the presence of oxygen, which produces light and oxyluciferin. This proposed enzymatic pathway is similar in many ways to the bacterial bioluminescent pathway except that the fungal system is not stimulated by the addition of reduced flavin mononucleotides, adenine dinucleotides, or dodecanal. Airth and Foerster successfully repeated their experiment with different combinations of enzymes and substrates obtained from A. mellea, C. velutipes, and P. stipitcus, suggesting that both substrate and enzyme are essentially the same for all bioluminescent fungi species (Desjardin et al., 2008).
During the early 1970s to mid 1990s, many researchers claimed to have isolated the chemical structure of fungal luciferin. Authors such as Endo and Isobe declared the unknown luciferin to be molecules such as lampterol, ergosta-4,6,8(14),22-tetraen-3-one, riboflavin, and lampteroflavin extracted and purified from O. japonicas (Desjardin et al., 2008). Although similar to the fluorescent spectra of fungal bioluminescence (around 530 nm), no further evidence suggests the involvement of any of these molecules in the mechanism of fungal bioluminescence in vivo.
Shimomura’s Hypothesis: Non-enzymatic Pathway
Shimomura isolated a sesquiterpene known as panal from fruiting bodies of P. stipticus (Nakamura et al., 1988). He suggested that panal was involved in the bioluminescent pathway of fungi. Panal is present in the form of its decanoic and dodecanoic esters, PS-A and PS-B (Shimomura, Satoh, and Kishi, 1993). When panal was incubated with ammonium salts or primary amines for 24 h, the pyrrolic derivative that formed showed light emission upon addition of iron(II) ions and hydrogen peroxide at ph 7-8.
From this data, Shimomura proposed a non-enzymatic mechanism to explain fungal bioluminescence where no luciferase was involved and panal was the luciferin precursor (see Fig. 2).
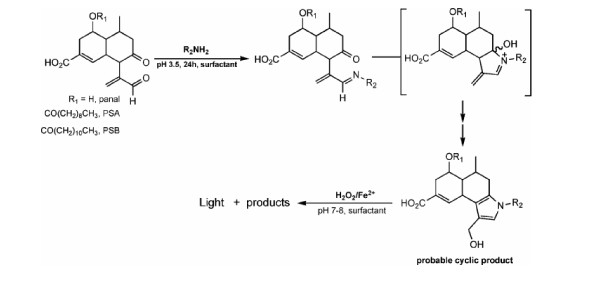
Figure 2. Shimomura’s Proposed Pathway for Fungal Bioluminescence
Due to panal’s low conjugation and high flexibility, panal becomes more rigid via a cyclization reaction which increases its fluorescence. This mechanism is not commonly accepted by scientists since the hydroxyl radicals produced in the presence of Fe2+ and H2O2 are highly oxidizing, which is not a physiologically plausible condition for bioluminescence.
Conclusion
The “natural” advantage of bioluminescence is mostly unclear. Evidence suggests that it is simply a byproduct of oxidative reactions. Based on Dubois’s studies, Airth and Foerster proposed a two-step mechanism for fungal bioluminescence involving an NADPH-dependent reductase and a fungal luciferase. In contrast, Shimomura suggested a non-enzymatic mechanism with an absence of luciferase and argued instead that panal was the luciferin precursor.
Like the green fluorescent protein found in jellyfish, the fluorescent genes in fungi can target genes in other organisms and tag their locations. This could be useful in pharmaceutical development, medical research, and gene therapy. Bioluminescent fungi can also be a biosensor. Manipulating fungi to glow in the absence of water would help prevent overwatering, reducing water use. Lighting streets with bioluminescent fungi would save energy and money. Bioluminescent fungi could also be sold for aesthetic purposes. Further research involving the viability of bioluminescent fungi applications and the chemical structure of the fungal luciferin should definitely be considered.
Sources
Airth, R. L., Foerster, G. E., 1962. The isolation of catalytic components required for cell-free fungal bioluminescence. Arch Biochem and Biophys. 97, 567-573.
Airth, R. L., McElroy, W. D., 1959. Light emission from extracts of luminous fungi. Jour Bact. 77, 249-250.
Bjorn, L. O., Ghiradella, H., 2008. Bioluminescence. Springer.
Desjardin, D. E., et al., 2008. Fungi bioluminescence revisited. Photochemical & Photobiological Sciences. 7, 170-182.
Haddock, S. H. D., et al., 2010. Bioluminescence in the Sea. Annual Review of Marine Science. 2, 443-493.
Nakamura, H., et al., 1988. Panal: a possible precursor of fungal luciferin. Tetrahedron. 44, 1597-1602.
Oliveira, A. G., Stevani, C. V., 2009. The enzymatic nature of fungal bioluminescence. Photochemical & Photobiological Sciences. 8, 1416-1421.
Shimomura, O., et al., 1993. Structure and non-enzymatic light emission of two luciferin precursors isolated from the luminous mushroom Panelus stipticus. Journal of Bioluminescence and Chemiluminescence. 8, 201-205.
Weitz, H. J., 2004. Naturally bioluminescent fungi. Mycologist. 18, 4-5.
